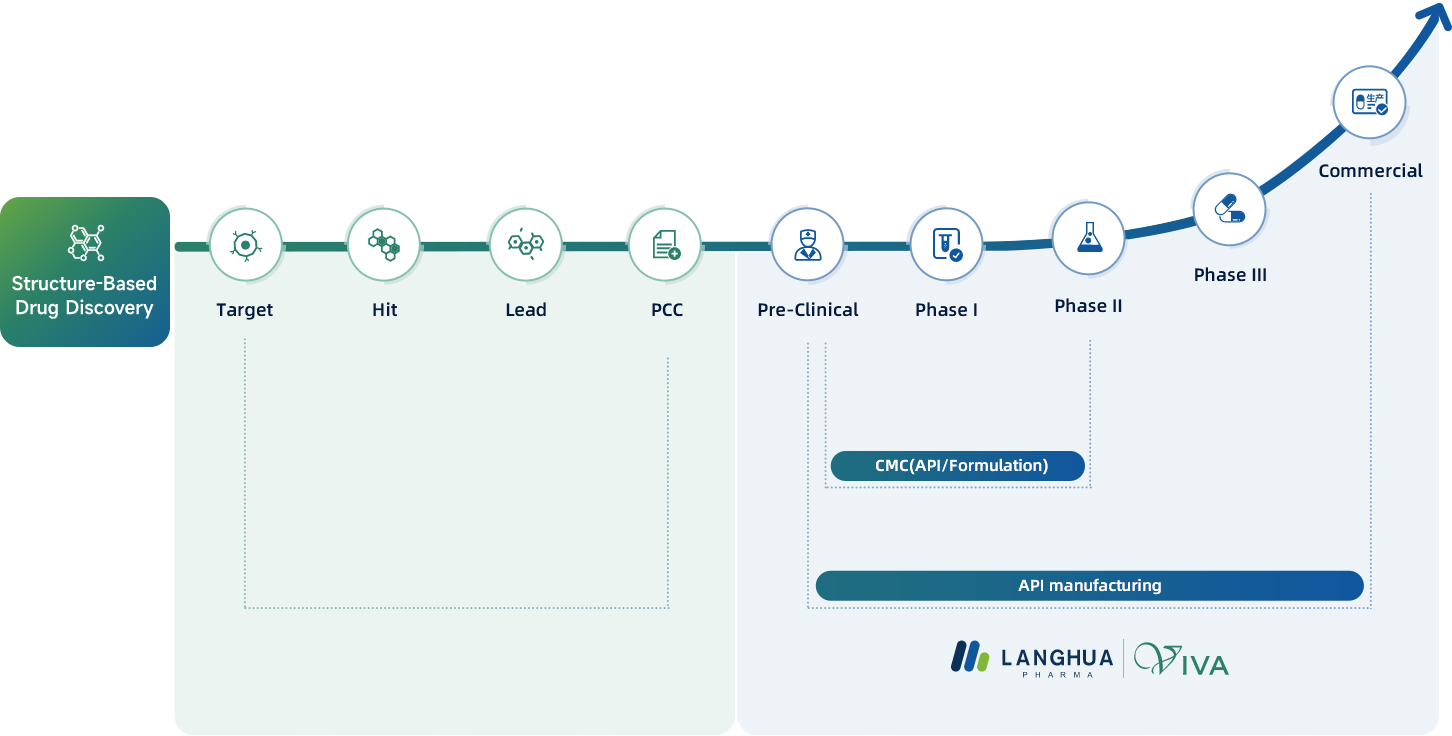
Langhua's innovative R&D network spans three strategic centers situated in Shanghai, Ningbo, and Taizhou, collectively offering over 13,000 square meters of advanced lab facilities. Our dedicated team of two hundred chemists, with over half holding esteemed PhD or Master's degrees, is well-equipped to drive breakthroughs in new drug development and CMC filing. Our field leaders bring a wealth of experience from renowned global pharmaceutical corporations, specializing in APIs & Drug Product development, scale-up processes, and expertly navigating IND & NDA filing procedures.
Our R&D centers are outfitted with cutting-edge lab equipment and analytical instruments, including NMR (Bruker-400 MHz), LC-MS (Agilent), TOF-MS (Agilent), SFC (Waters UPC2), GC-MS/MS (Waters), GC-MS/MS (Agilent), ICP-MS, LC-CAD, XRPD, IR, UPLC, and more. These state-of-the-art tools not only meet stringent regulatory standards but also play a pivotal role in driving effective API and Formulation development.